FDA Approves Convalescent Plasma To Treat The Coronavirus
Where's the Coronavirus cure? That's the question on everyone's mind. The pandemic known as COVID-19 has taken over the world and then some.
This article is more than 2 years old
Where’s the Coronavirus cure? That’s the question on everyone’s mind. The pandemic known as COVID-19 has taken over the world and then some.
Countries have closed off their borders virtually locking the public away to ease the transmission of the virus. Italy’s streets are pretty much empty as they are one of the hardest hit by the virus. Across the globe, as the number of infected increases at an alarming rate, more and more countries are limiting people and their movements. Sports and entertainment have come to a halt. Stores, shops, restaurants, movie theaters, and bars have shuttered. Grocery stores have been overrun by scared patrons and many stores find themselves unable to keep up with the demand.
So, with all this panic, with all the expected sickness to take over the globe, what are we and the rest of the world doing about it? Have scientists and researchers been burning both ends of the candle to come up with something, anything, that can slow down COVID-19 or perhaps even cure it? The hopeful answer is yes. A coronavirus cure is coming.
Here’s what we’re doing to find a coronavirus cure.
CONVALESCENT PLASMA
Medical researchers have begun to use the plasma, taken from the blood of those who are “convalescing” (have recovered) from COVID-19, to see if it will help fight the brutal virus. Plasma, which contains antibodies that fight diseases, is what remains of blood once the white and red blood cells, along with the platelets, are removed.
Now this convalescent blood plasma treatment has received approval for use from the FDA. In a statement issued August 23, 2020 they say, “it is reasonable to believe that COVID-19 convalescent plasma may be effective in lessening the severity or shortening the length of COVID-19 illness in some hospitalized patients,” adding, “The known and potential benefits of the product outweigh the known and potential risks of the product.”
That doesn’t mean the FDA believes this is a cure for COVID-19. They caution that it “does not yet represent a new standard of care based on the current available evidence.” So the FDA “continues to recommend that the designs of ongoing randomized clinical trials of COVID-19 convalescent plasma and other therapeutic agents remain unaltered.”
In a coincidence that is perhaps not a coincidence, the day before the FDA issued this approval President Trump tweeted: “The deep state, or whoever, over at the FDA is making it very difficult for drug companies to get people in order to test the vaccines and therapeutics. Obviously, they are hoping to delay the answer until after November 3rd. Must focus on speed, and saving lives!”
It’s worth noting that the U.S. Food and Drug Administration had already given the okay to allow researchers to request emergency authorization to test if plasma will help patients. That happened months ago. This move simply makes it a little easier and could be viewed as a largely symbolic way to answer Trump without actually doing anything new to help people suffering from the Coronavirus.
While there is hope for convalescent blood plasma as a real solution, the process has had some mixed history in terms of success. Plus, it’s a time-consuming, it is very expensive and can be difficult to administer on a large scale. In the past, though, convalescent plasma has seen success when used in outbreaks of such diseases like polio, mumps, and measles, which is why this treatment holds such hope.
CORONAVIRUS VACCINES BEING DEVELOPED AS CURES
Operation Warp Speed

On Friday May 15, 2020 President Donald Trump announced a new public-private partnership to develop a vaccine for COVID-19. They’re calling it “Operation Warp Speed” a name which references the way ships travel through space in the fictional universe of Star Trek.
Of Operation Warp Speed President Trump says, “This is an endeavor unlike anything our country has seen since the Manhattan Project…No one has seen anything like we’re doing now within our country since the second world war. Incredible.”
Though most experts seem to think a virus is at least a year and a half off, or maybe even impossible, Trump said this of Operation Warp Speed’s efforts: “It’s objective is to finish developing and then to manufacture and distribute a proven coronavirus vaccine…prior to the end of the year. I think we’re going to have some very good results coming out very quickly.”
Moncef Slaoui, the former head of GlaxoSmithKline’s vaccines division will oversee Operation Warp Speed’s vaccine development. Army Gen. Gustave Perna will oversee logistics as OWS chief operating officer.
The Hank-cine

To help develop a vaccine scientists need blood donations from people who have recovered from COVID-19. Among those who have donated: Tom Hanks.
Hanks was one of the earliest people to contract the Coronavirus so it makes sense that he might be one of the first choices to help cure it. During an interview with NPR Tom Hanks revealed that he and his wife Rita Wilson reached out to try and offer their help saying, “We have not only been approached, we have said, ‘Do you want our blood? Can we give plasma?” Their offer was accepted and now Tom Hanks blood is a pivotal part of developing a Coronavirus cure.
Should Tom’s blood prove to be the key he has already suggested a name for the vaccient that might result. He’s (tongue-in-cheek) hoping they’ll call it The Hank-cine.
Johnson & Johnson’s Coronavirus Vaccine

Pharmaceutical mega-corp Johnson & Johnson announced on March 30, 2020 that they’ve developed a vaccine which they believes shows promise for halting the spread of COVID-19. They hope to begin human trials with the vaccine as early as September of 2020.
Johnson & Johnson’s CEO Alex Gorsky appeared on NBC’s Today Show and revealed “We have a candidate that has a high degree of probability of being successful against the COVID-19 virus. We’ve got the production capabilities to be able to ramp up production of this in a relatively short period of time so it can become available”
Johnson & Johnson has partnered with Biomedical Advanced Research and Development Authority to fast track development of their vaccine. If it works as they hope it will, the anticipate having it ready for widespread use in early 2021. They plan to produce a billion doses for use worldwide.
Gorsky says, “Literally within the next few days and weeks, we’re going to start ramping up production of these vaccines as well and we should be able to have several hundred million doses available by the middle of next year. Our goal is to have a billion prepared by the end of 2021”
If 2021 sounds like it’s a long time to wait, it’s not a long time to develop a vaccine. If they’re ready to deliver by then, it will be an almost record-setting pace. The development of a vaccines usually takes five to seven years. Johnson & Johnson is proposing to do it in under one year by investing over $1 billion in research, development, and testing.
CHINA’S COVID-19 VACCINE CLINICAL TRIAL
China has given the green light go on a new clinical trial for a vaccine that was developed by researchers who are led by a biowarfare expert. This unnamed vaccine was created by the Academy of Military Medical Sciences in Wuhan and approved to be used as a coronavirus cure by National Medical Products Administration.
Team leader Chen Wei, a top military biowarfare expert, said, “We are a community of shared future for mankind, and vaccine is one of the most powerful scientific and technological weapons to end the novel coronavirus epidemic.” She also stated that she and her team are fully prepared for large-scale production of the vaccine. “In accordance with international standards and domestic laws and regulations, we have made preliminary preparations for its safety, effectiveness, controllable quality and mass production,” Chen said, per the New York Post.
AN INVESTIGATIONAL VACCINE TO CURE CORONAVIRUS

Kaiser Permanente Washington Health Research Institute in Seattle has started a clinical trial to evaluate an investigational vaccine that has been designed to protect against coronavirus. Doctors are taking 45 healthy adults ranging in age from 18-55 and giving them different doses of the vaccine, mRNA-1273, for safety and to see its ability to prompt an immune response in the participants.
“Finding a safe and effective vaccine to prevent infection with SARS-CoV-2 is an urgent public health priority,” said National Institute of Allergy and Infectious Diseases Director Anthony S. Fauci, M.D. “This Phase 1 study, launched in record speed, is an important first step toward achieving that goal.”
The fight to contain and control and hopefully cure the coronavirus is on. Researchers around the world are combining their efforts as they look for the solution to this pandemic. The results so far look promising but it’s obviously a work in progress. Continue to be vigilant in how you protect yourself and others while doctors and scientists pull together to knock out COVID-19.
Other Coronavirus Vaccines In Development
At the present time, there is not one vaccine approved for COVID-19. The research for a vaccine is moving along quickly but some things still need time.
Adenovirus Type 5 vector (Ad5-nCoV): An adenovirus is a common virus that leads to pneumonia or bronchitis. Extensive studies have been on-going for years for vaccines that will simulate the production of protective antibodies. A vaccine of this nature is being tested by CanSino Biologics and Johnson & Johnson.
RNA; LNP-encapsulated mRNA (mRNA 1273): This hopeful vaccine is built on previous research into the MERS virus. This is a messenger RNA vaccine where a portion of the virus’s genetic material is injected into your muscle. The RNA then carries this genetic information from DNA need to make proteins, telling the cells how to make this protein to fight the virus.
Drugs As Possible Cures
CURING COVID-19 WITH REMDESIVIR
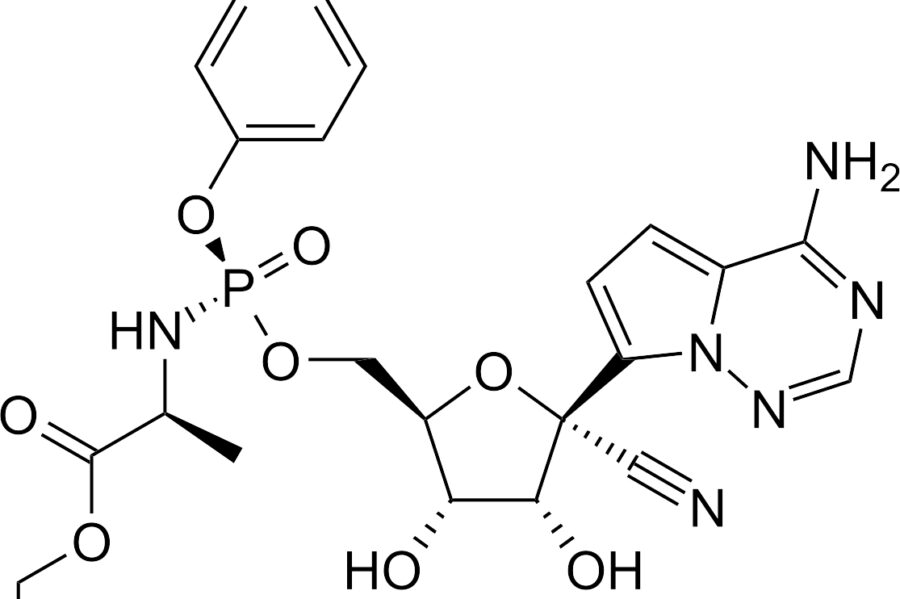
Whitehouse medical expert Dr. Fauci has announced that studies indicate remdesivir works to block the Coronavirus. Fauci says, “the data shows remdesivir has significant, clear-cut, positive effect on diminishing the time to recovery.” He continued, “what it has proven is that a drug can block this virus… This drug happens to be blocking an enzyme that the virus uses.”
Here’s Dr. Fauci’s announcement…
Nearly a decade ago, a group of chemists got together and created a compound they simply called 3a. This compound, in experiments, was able to fight off a number of different viruses. One of the viruses was a coronavirus. A descendent of this compound, remdesivir from Gilead Sciences, is being used for patients with COVID-19 in hopes it can do to this virus as it did ten years ago. Gilead’s initial plan for the drug was to combat another deadly disease, Ebola.
Remdesivir is has beenand is being tested in five different coronavirus cure clinical trials. Some analysts are hinting at concerns from the drug as tiny bits of information has been coming out from these trials. But for others, there is hope. So much so that Bruce Aylward of the World Health Organization is standing by the drug. “There’s only one drug right now that we think may have real efficacy. And that’s remdesivir.”
Many researchers agree that finding a drug that works is typically a lengthy process of trials and more trials. Gilead’s vice president of virology, Tomas Cihlar, said in an interview with STAT, “Drug discovery and development is usually a very long and tedious process and you could have many failures on the path to an approved product.” He also spoke about remdesivir’s chances with COVID-19, “It would be wonderful if it works. But it needs to be proven.”
The hope for this drug as a coronavirus cure came early when a 35-year old man caught COVID-19 after a visit to family in Wuhan, China. When he returned home to Washington, he began to feel the deadly effects of the disease. George Diaz, the infectious disease section chief at Providence Regional Medical Center in Everett, Washington got in touch with the Centers for Disease Control and Prevention and it was they who suggested to Diaz to try Gilead’s remdesivir.
Because the drug has not yet been approved, the Food and Drug Administration had to give the okay to treat the patient through the compassionate use program, which allows the treatment of unapproved drugs to be used in select circumstances. The patient was given an IV of the drug and amazingly began to feel better the very next day. “We were aware that he was the first patient on the planet getting the drug for this infection, so we were super interested to see, hopefully, if he would improve,” Diaz said.
CURING CORONAVIRUS WITH CHLOROQUINE
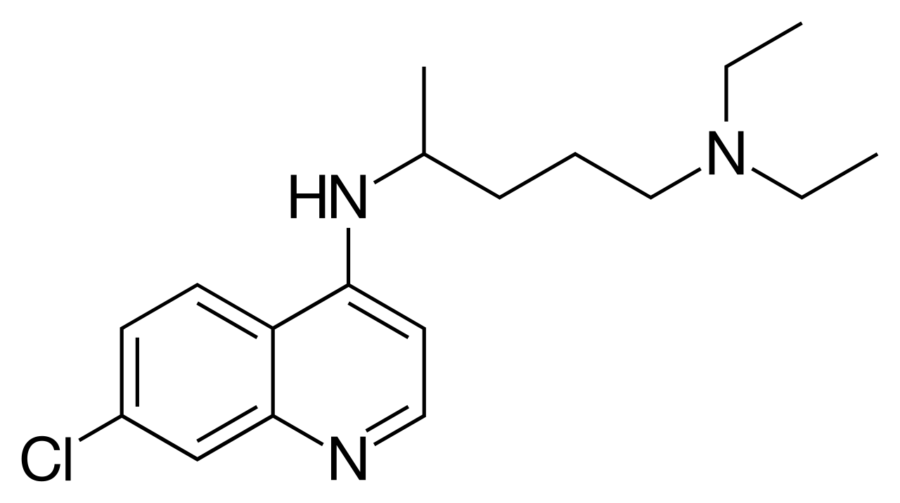
One drug that has shown signs of combating this version of the coronavirus is Chloroquine. The drug has been around since 1934 when Hans Andersag discovered it. Chloroquine is primarily used for the prevention and treatment of malaria. It is also used for amebiasis (or amoebic dysentery), rheumatoid arthritis, and lupus erythematosus.
The FDA has now issued an emergency authorization for medical professionals to prescribe both Chloroquine and Hydroxychloroquine to treat the novel Coronavirus COVID-19.
The Department of Health and Human Services issued this guideline for its use saying hydroxychloroquine and chloroquine can, “be distributed and prescribed by doctors to hospitalized teen and adult patients with Covid-19, as appropriate, when a clinical trial is not available or feasible.”
Meanwhile Forbes reports that 30 million doses of hydroxychloroquine have been added to the United States federal government’s supply of medical supplies specifically set aside for emergencies. Should the health community start to gain more confidence that chloroquine really is working, they’re ready to start handing it out.
The FDA’s approval does not mean that Chloroquine has been proven to cure the Coronavirus. As of yet there is still only anecdotal evidence of its efficacy. However, with the pandemic spreading Doctors are willing to try more things, and this gives them the ability to try it without getting caught up in red tape.
Early reports of using this drug for those with COVID-19 are showing encouraging signs of being a coronavirus. A group of French researchers published in the International Journal of Antimicrobial Agents dated February 15, 2020, “If clinical data confirm the biological results, the novel coronavirus-associated disease will have become one of the simplest and cheapest to treat and prevent among infectious respiratory diseases.”
One author of this article, Didier Raoult, is a prominent infectious-disease expert who is testing a version of the drug called hydroxychloroquine in a clinical trial with a few dozen patients with COVID-19. Early reports on this trial have also been positive.
A combination of this drug with the antibiotic azithromycin have also been shown to reduce the viral load in his clinical trial patients. Azithromycin is used to fight bacteria which includes respiratory infections and Medscape reported that this drug may also play a role in fighting COVID-19.
Steven Seedhouse is a biotech analyst at Raymond James. In his opinion, chloroquine presented just as promising as coronavirus cure as any other treatment option now being used for COVID-19. This also includes remdesivir, which is an antiviral drug that biotech giant Gilead Sciences has been developing and that officials from the World Health Organization have called “the most promising candidate” for COVID-19 treatment. In Seedhouse’s estimation, “If we had to pick one of the three at this point that actually seems most likely to have the biggest impact on treating COVID-19 in the coming months/years, it would be chloroquine.” In fact, Tesla and SpaceX boss Elon Musk tweeted out his thoughts on chloroquine stating, “Maybe worth considering chloroquine for C19.”
JAPAN’S ANTIVIRAL CURE
According to The Guardian, a drug Japan uses to treat the influenza is being used on COVID-19 patients. It’s an antiviral drug called Favipiravir and is showing positive results in clinical trials involving 340 patients from the Wuhan and Shenzhen in China. The drug, developed by Fujifilm Toyama Chemical, has been quite effective according to Zhang Xinmin, an official at China’s science and technology ministry. “It has a high degree of safety and is clearly effective in treatment,” Zhang said.
UK’S EXPERIMENTAL LUNG DRUG
Synairgen, a UK biotech firm, is ready to trial its experimental lung drug, SNG001 on curing the coronavirus. The drug, which is inhaled, was first developed to treat chronic obstructive pulmonary disorder (COPD), but this has been put on pause so they can concentrate on COVID-19.
During their trial, half the patients will receive SNG001 while the other half will get a placebo. This will give doctors a clear indication of what their drug can accomplish.
Other Antivirals Being Tested
Arbidol (umifenovir): This antiviral blocks virus entry into healthy cells. It has not been approved by the FDA but is available in Russia and China. China is observing its potential.
ASC09: Another HIV drug, this is a protease inhibitor that prevents new viruses from maturing and invading the healthy white cells. Hopes are that this drug can do the same for COVID-19 patients.
Azvudine: This drug is an inhibitor that blocks an enzyme, thus not allowing the virus to replicate.
Ganovo (danoprevir): This drug was first used to treat hepatitis C. It has shown to inhibit the virus’s protease so it can’t replicate. The hope is it will do the same for COVID-19 patients.
Prezcobix (darunavir): Another protease inhibitor that is being used in HIV patients. It is used in combination with a pharmacokinetic enhancer, which slows the breakdown of Prezcobix, allowing it to remain in the body longer. A study is being conducted on COVID-19 patients to see if this treatment will work.
Truvada (emtricitabine and tenofovir): This combination is used to reduce the risk of HIV infection. They work in different ways in stopping HIV replication so the hope here is they will do the same for the coronavirus.
Xofluza (baloxavir marboxil): The enzyme which causes flu virus replication is called Endonuclease. Xofluza is a polymerase acidic endonuclease inhibitor that is FDA approved and now being used to help treat COVID-19 patients with pneumonia.
Other Drugs Being Tested As Coronavirus Treatments
Monoclonal antibodies are molecules made in a lab to fight cancer or ebola which work as “substitute antibodies” that boost or mimic the body’s immune system so it can attack the virus.
Actemra (tocilizumab): This rheumatoid arthritis drug blocks interleukin-6 (IL-6) and keeps it from attacking healthy tissue in an overreacting immune system. It has helped several critical COVID-19 patients.
Avastin (bevacizumab): This vascular endothelial growth factor blocker is being trialed in China to assess its effectiveness for treating shortness of breath.
Gimsilumab: Acute respiratory distress syndrome (ARDS) is found in COVID-19 patients, giving their lungs the inability to fully inflate. Gimsilumab will be used to fight ARDS.
Leronlimab (PRO 140): This drug is used in the fight against HIV. It binds itself to the CCR5 receptor, which stops the release of inflammatory cytokines. It’s in clinical trial to test the effects it will have on serious COVID-19 patients.
PD-1 and Thymosin: This combination is in a clinical trial in China to study how well they fight severe pneumonia in COVID-19 patients.
Sylvant (siltuximab): Doctors in Italy are studying this drug in hopes that it will help reduce inflammation in the lungs to those with severe respiratory disorder.
TJM2 (TJ003234): This drug acts similar to Gimsilumab, so it is another hopeful weapon against acute respiratory distress syndrome.
APN01: This drug has been created to treat acute lung injury, pulmonary arterial hypertension and acute respiratory distress syndrome. It has been shown to lessen lung damage in mice, it is now being clinical trialed in hopes it will have the same effect on humans.
TESTING

Nearly everyone agrees that the biggest hurdle to getting COVID-19 under control is adequate and widespread testing. That goal of testing everyone just took a huge step forward on April 22, 2020 when the FDA has finally authorized a direct-to-consumer test for the virus. The test, which is being called Pixel, is created by health services giant LabCorp. The cost for the at-home test is going to run a pricey $119 but includes two-way overnight shipping and physician services from PWNHealth.

It is obvious that LabCorp’s initial desire is to get first responders and health care workers the testing first, per president and CEO of LabCorp, Adam Schechter. “LabCorp continues to develop new ways to help patients and healthcare providers fight the COVID-19 crisis through our leading testing capabilities and deep scientific and research expertise. Our at-home collection kits are designed to make it easier and safer to test healthcare workers and first responders during this important time.”
The self-collection kit will have everything one needs to collect your sample and send it off, including easy to understand instructions. The results of your test will be given to an online website. LabCorp has also put together a nasal sample collection video. No exact date has been given to when the tests will be available but look for them soon.
Cell Therapies Being Tested
Mesenchymal stem cells (MSC): Not only can stem cells self-renew, they can also differentiate into different types of cells. MSCs can be taken from humans (adults) or animals and have been used in research on damaged lungs.
MultiStem: Similar to MSC, MultiStem fights damaged lungs. It has already been through an early-stage clinical trial and has shown patients to come off ventilators faster than those who didn’t receive treatment and also lower mortality rates.
WHAT THE NOVEL CORONAVIRUS LOOKS LIKE
The list of primary symptoms for COVID-19 is short and sweet. Fever, cough, and shortness of breath. But a new study that was published in the American Journal of Gastroenterology now suggests that some people who have contracted the coronavirus have experienced digestive symptoms, such as diarrhea, as one of the first signs of the virus. Other than those primary symptoms, they become worse if the virus worsens. It turns into chest pain, pneumonia and difficulty breathing.
The new study looked at data taken from 204 patients with COVID-19 from China’s Hubei province showed that 48.5% of them arrived with some type of digestive symptoms such as vomiting, diarrhea, or abdominal pain.
These research findings can help doctors as they try cure the coronavirus before it gets to the critical stages. Brennan M.R. Spiegel, M.D., the co-editor-in-chief of the American Journal of Gastroenterology said in a press release that being aware of these digestive symptoms can be huge in detecting COVID-19 earlier. “In this study, COVID-19 patients with digestive symptoms have a worse clinical outcome and higher risk of mortality compared to those without digestive symptoms, emphasizing the importance of including symptoms like diarrhea to suspect COVID-19 early in the disease course before respiratory symptoms develop. This may lead to earlier diagnosis of COVID-19, which can lead to earlier treatment and more expeditious quarantine to minimize transmission from people who otherwise remain undiagnosed.”
NOTHING HAS BEEN APPROVED

Before we jump into that “hopeful answer” for a coronavirus cure, let’s be clear that at this precise time (March 19, 2020 when I’m writing this) there are no approved treatments or vaccines. But, with that said, there are drugs out there showing promise as they are tested to treat patients with COVID-19. There are also some clinical trials kicking off that could provide relief for sufferers of the Coronavirus.
That means people should not go off half-cocked and start experimenting on themselves. Yet as people have heard about how promising the drug chloroquine is, they’ve started trying to create their own, with disastrous consequences.
For example, an Arizona couple in their sixties ingested fish tank cleaner as a Coronavirus cure. As relayed by NBC they mixed a small amount of the substance with a liquid and drank it, thinking it might work as Coronavirus preventative. The husband is now dead. The wife survived after calling 911 when she started vomiting and her husband began to develop respiratory problems.
One of the ingredients in some fish tank cleaner tablets is indeed chloroquine, the malaria drug being used to treat the Coronavirus. However the tablets also contain other ingredients and, obviously, are not intended to be ingested. If you swallow one, they’re deadly.











